1. Why do atoms bond? (There is a priority that all atoms have. They bond to fulfill this need… what is it?) ___Atoms love to have their outer layer (Valence level) of electrons full. They will give or share electrons to make this happen._____
2. Describe the process for each type of bond:
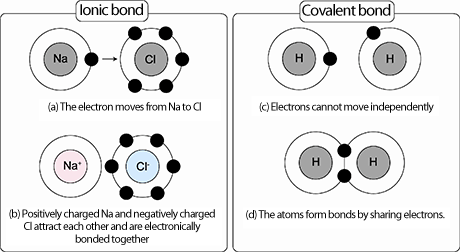
- Ionic __In an ionic bond, an atom with very few electrons in the outer (valence) level will give an electron(s) to an atom that is only missing 1 (or a couple) electrons in its valence level. When the electron is given, the one atom becomes positive and the other becomes negative. The oppositely charged atoms (called ions) will attract each other. This is called bonding.________________
- Covalent ____In a covalent bond, two atoms share electrons. The shared electrons travel around both nuclei connecting them with a covalent bond.____
3. Give 2 characteristics for molecules that are bonded in these ways
- Ionic _____(1) conducts electricity (2) crystalline (3) high boiling point ____
- Covalent _____ (1) does not conduct electricity (2) lower melting point _____________
No comments:
Post a Comment
Thank you for commenting on our science class blog :)
Mrs. Tiday